Central Imaging Lab
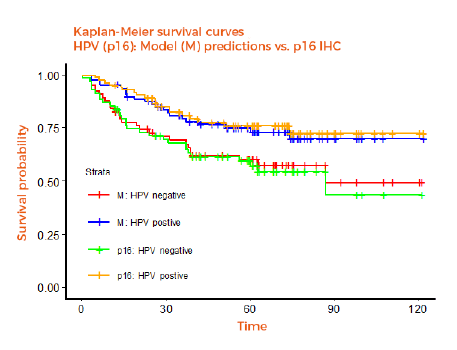
Medical images are used in many therapeutic areas and contain a goldmine of information. Part of this information is traditionally extracted by radiologists and with the advances made in image processing and artificial intelligence, computers are now able to go deeper in the extraction of almost every detail which is hidden in the image. In clinical trials, centralized and automated analysis of medical images allow the removal of human-induced variability and bias. Thanks to our extensive experience in handling medical imaging variability, applying AI and trial statistics, and working with pharma companies, our goal is to position Radiomics.bio as the next generation imaging CRO. For this, Radiomics.bio provides a full package of services.
QC
CDISC is an organisation developing standards used for the different steps of a clinical trial including analyse and submission.
DICOM is the international standard used for the medical images, including transfer and storage.
This allows us to respect FAIR principles (Findable, Available, Interoperable, Reusable) regarding data.
Moreover, Radiomics.bio helps its clients with the:
- Set-up of clinical trials
- Providing optimal image acquisition protocol for high quality data
- Imaging analysis phase:
- Scans are free of confound information such as artifacts
- Scans contain the correct region of interest (ROI)
- Scans are acquired following appropriate acquisition protocols.
Segmentation
The first step in the image analysis process is the segmentation of the region of interest (ROI). Segmentation can be performed:
- Manually
- Fully automated
- Combination of both
Results are always validated by at least one board-certified radiologist.
Our R&D team continuously builds automatic segmentation tools. These solutions can replace the manual segmentation once their accuracy is demonstrated to be non-inferior to a set of board-certified radiologists.
Currently we have developed automatic segmentation tools for the following:
Radiomics
Interpretation & Reporting
At Radiomics.bio we know that data alone is not enough to lead to concrete solutions addressing the unmet medical needs of our clients. The high-resolution dataset generated by our radiomics analysis requires adapted trial statistics approaches. Therefore, our team of experienced data scientists and statisticians is specialized in guiding our clients during the trial data analysis steps, providing data interpretation, and reporting at different levels for scientific, executive, and market-oriented audiences. This will guarantee that data is used and presented in optimal ways, to make sure that our conclusions are insightful but not overly optimistic.