- February 16th, 2022
- Category: news
9 in 10 pivotal clinical trials in oncology fail: Radiomics can help to make the best use of research investments
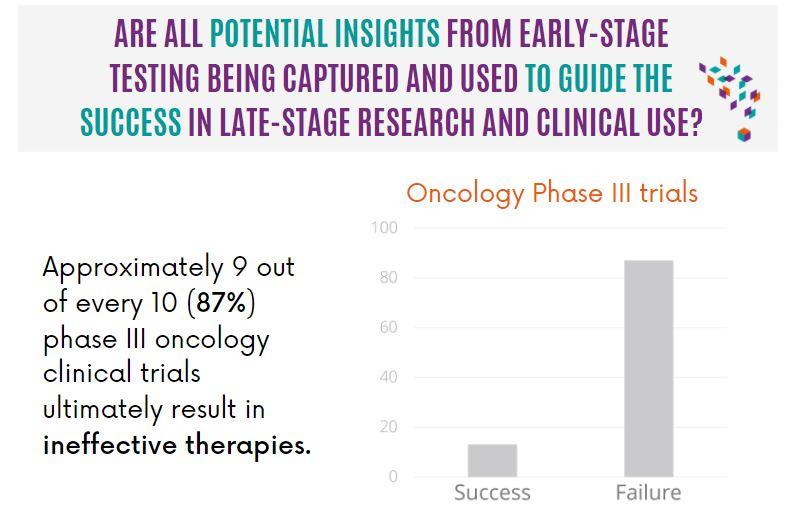
A recent review showed that 9 out of 10 clinical trials fail to test the therapy as effective, this begs the question if all potential insights from early-stage testing are being captured and used to guide the success in late-stage research and clinical use?
Blogpost
9 in 10 pivotal clinical trials in oncology fail: Radiomics can help to make the best use of research investments

Late-stage clinical development and phase III trials are designed to generate evidence which supports the claim that the benefit of the therapy outweighs any potential risks. This evidence is then taken to the relevant bodies to inform regulatory and reimbursement decisions. A recent review by Shen et al.1 highlighted a highly problematic trend in oncological clinical trials – too many pivotal phase III trials test therapies that are ultimately ineffective. This review, which considered a decade’s worth of trials in the US, found that 87% of those that used Overall Survival (OS) as the primary endpoint, determined the drug as an ineffective therapy.1 Ineffective therapies have significant costs for pharmaceutical sponsors, the healthcare system in general, and ultimately for patients. Reducing this failure rate is a recognized and prioritized grand challenge in medicine.
Facing up to this grand challenge begs the question, are all potential insights from early-stage testing being captured and used to guide the success in late-stage research and clinical use?
For the purpose of this blogpost, the term ‘ineffective therapies’ encompasses two different underlying reasons as to why the therapy is ineffective. First, a therapy can achieve statistical significance in the trial but not reach the target effect size or benefit. In this case, it would be worthwhile to explore in earlier stages if there are more sensitive measures of treatment response that could help to ensure that the therapy also shows clinical benefit. Secondly, therapies can have neither clinical benefit nor the target effect size for benefit. These therapies can still yield valuable information and using the right insights can help to identify (a minority) responder and non-responder cohorts. In both cases, radiomics can provide imaging-based insights throughout early-stage development to support and guide the clinical development process.
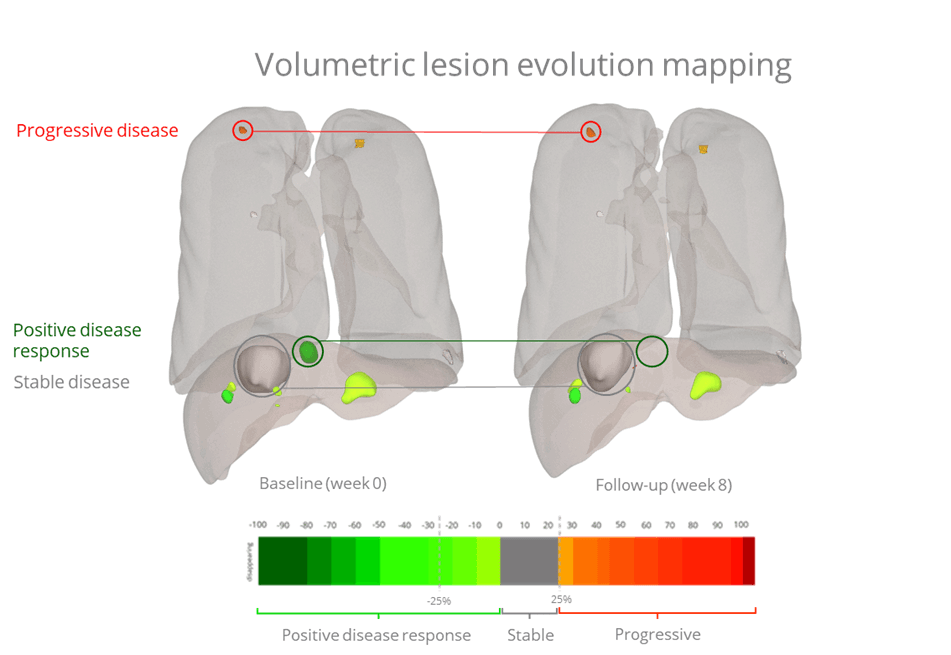
As we continue to learn more about the variability of tumor genetic expression (inter/intra-tumoral heterogeneity), therapy resistance and local pharmacokinetics, targeted therapies are becoming more and more popular. This has also driven drug developers to explore therapy evaluation alternatives that go beyond the current FDA requirements. One such avenue is looking at each individual lesion to identify and characterize the lesions (and therefore the patients) that will be likely to respond to therapy. As can be seen in Figure 1, even within the same treatment, there can be tumors that positively respond, tumors that exhibit stable disease, and tumors that will progress (Fig. 1 – Radiomics internal analysis).
Using advanced image analysis to investigate each measurable lesion (entire disease burden) can also increase statistical analysis power. This is especially true in early phase clinical trials, where the statistical power will increase significantly by considering all measurable lesions for therapeutic evaluation, rather than only considering those deemed target according to various guidelines.2 For example with RECIST 1.1., only a maximum of 5 lesions per patient and 2 per organ are considered, which limits the information that is being used to build an understanding of the treatment response. Characterizing tumors using quantitative measures of size, shape, texture, and intensity also provides a sensitive means of identifying novel imaging biomarkers, and a way to identify responders, even within a population that is considered non-responsive overall. Another application of this type of analysis is to characterize the tumor, again in different dimensions of size, shape, texture, and intensity, and across multiple timepoints. Such ‘tracking’ can provide additional insights into whether the response could be improved further by increasing the dose or combining different treatments or therapies.
A radiomic analysis can also be used to explore the correlation between lesion response (local) and patient response (global), so that phase III trials can be designed to recruit the “right” patients, those that are phenotypically more likely to respond and therefore maximize the compound value. Characterized lesions are correlated against accepted indicators like Overall Survival (OS) and Progression Free Survival (PFS), or clinical co-variants such as genetic expression or disease status (i.e., HPV3, PD-L14,5, EGFR6,7etc.). These insights can also be used to ensure that trial cohorts are balanced at baseline between treatment arms, providing a fair chance for success and avoiding unnecessary failure.
If we go back to the question if all potential insights from early-stage testing are captured and used, it can be concluded that it is definitely possible to gain useful insights throughout early-stage clinical trials, and to use these to reduce the proportion of ineffective therapies that make it through to later stages. Nevertheless, capturing these insights will require to plan correctly in the early phases and make the correct adjustments. Supplementing clinical trials with a radiomics analysis is a cost-effective way to gain more from the imaging data that is already being collected. These insights go beyond what is required by the FDA and similar regulatory bodies. It can provide more sensitive measures of treatment response, a more balanced trial design, the ability to identify likely responders from baseline imaging, and a higher chance to push through a treatment to phase III that has a higher likelihood of resulting in an effective treatment. Ineffective therapies, whether they show clinical benefit or not, have significant costs for drug developers, patients, and the entire healthcare system. To progress oncological research forward and ensure that we can provide the right therapies to the right patients, we need to implement novel insights such as these to guide our decision making.
About Radiomics
Founded in 2016 and based in Liège, Radiomics is a Belgian AI powered research organization for next-generation image analysis, based on the unique experience of its founders, pioneers in radiomic science. Oncology being radiomics initial focus area, we have developed promising applications in various other therapeutic areas, such the respiratory field. Radiomics uses its advanced image analysis technology based on AI, deep learning, machine learning, and federated learning to obtain quantitative biomarker measurements from medical imaging, in a repeatable, reliable, and relevant manner. Its goal is to support decision-making through insights and optimize pharmaceutical and biotech companies’ clinical trials and drug development studies and provide clinicians with a patient-centered approach based on personalized medicine.
Want to know more after reading this article?
Check out the other parts of the Companion Diagnostics series (https://radiomics.bio/whitepapers/) or contact us via [email protected]
References
[1] Shen, C., Ferro, G., Xu, H., Kramer, D. B., Patell, R. & Kazi, D. S. (2021). Underperformance of contemporary phase III oncology trails and strategies for improvement. Journal of the National Comprehensive Cancer Network, 19(9), 1072-1078.
[2] Lambin, P., Leijenaar, R. T. H., Deist, T. M., Peerlings, J., de Jong, E. E. C., van Timmeren, J., Sanduleanu, S., Larue, R. T. H. M., Even, A. J. G., Jochems, A., van Wijk, Y., Woodruff, H., van Soest, J., Lustberg, T., Roelofs, E., van Elmpt, W., Dekker, A., Mottaghy, F. M., Wildberger, J. E. & Walsh, S. (2017). Radiomics: thee bridge between Medical Imaging and Personalized Medicine. Nature Reviews Clinical Oncology, 14(12), 749-762.
[3] Leijenaar, R. T. H., Bogowicz, M., Jochems, A., Hoebers, F. J., Wesseling, F. W., Huang, S. H., Chan, B., Waldron, J. N., O’Sullivan, B., Rietveld, D., Leemans, C. R., Brakenhoff, R. H., Riesterer, O., Tanadini-Lang, S., Geckenberger, M., Ikenberg, K. & Lambin, P. (2018). Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol, 91.
[4] Jiang, M., Sun, D., Guo, Y., Xiao, J., Wang, L. & Yao, X. (2020). Assessing PD-L1 expression level by radiomic features from PET/CT in nonsmall cell lung cancer patients: an initial result. Academic Radiology, 27(2), 171-179.
[5] Yoon, J., Suh, Y. J., Han, K., Cho, H., Lee, H. J., Hur, J. & Choi, B. W. (2020). Utility of CT radiomics for prediction of PD-L1 expression in advanced lung adenocarcinomas. Thoracic cancer, 11(4), 993-1004.
[6] Hong, D., Xu, K., Zhang, L., Wan, X. & Guo, Y. (2020). Radiomics signature as a predictive factor for EGFR mutations in advanced lung adenocarcinoma. Frontiers in oncology, 10(28).
[7] Rossi, G., Barabino, E., Fedeli, A., Ficarra, G., Coco, S., Russo, A., Adamo, V., Buemi, F., Zullo, L., Dono, M., De Luca, G., Longo, L., Giovanna Dal Bello, M., Tagliamento, M., Alama, A., Cittadini, G., Pronzato, P. & Genova, C. (2021). Radiomic detection of EGFR mutations in NSCLC. Cancer Research, 81(3), 724-731.


